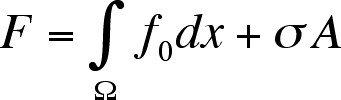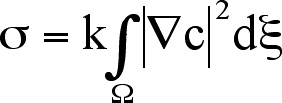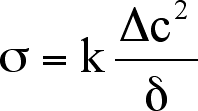The interfacial tension between immiscible fluids is an equilibrium thermodynamic
property that results from the differences in intermolecular interactions between
the two different types of molecules. When two miscible fluids are brought in
contact, a large concentration (and density) gradient can exist, which relaxes
through diffusion as the system approaches the uniform, equilibrium state. Because
the molecules have different structures, there are necessarily differences in
intermolecular interactions, which may lead to transient interfacial phenomena.
For most immiscible systems an infinitely narrow interface, consisting of a discontinuous
jump in concentration, was an accurate description. However, for polymer systems
and systems near their critical point an infinitely narrow transition zone does
not apply. (Nor does it apply to the transition zone between two miscible fluids.)
Van der Waals was the first to propose an alternate definition of an immiscible
interface [1]. He assumed that the interface consisted of a continuous region,
in which there was a transition in concentration from one fluid to the other.
Fig. 1 depicts such a transition zone, with increasing concentration from right
to left. The interface is then also defined by the width, d, of the transition
zone.
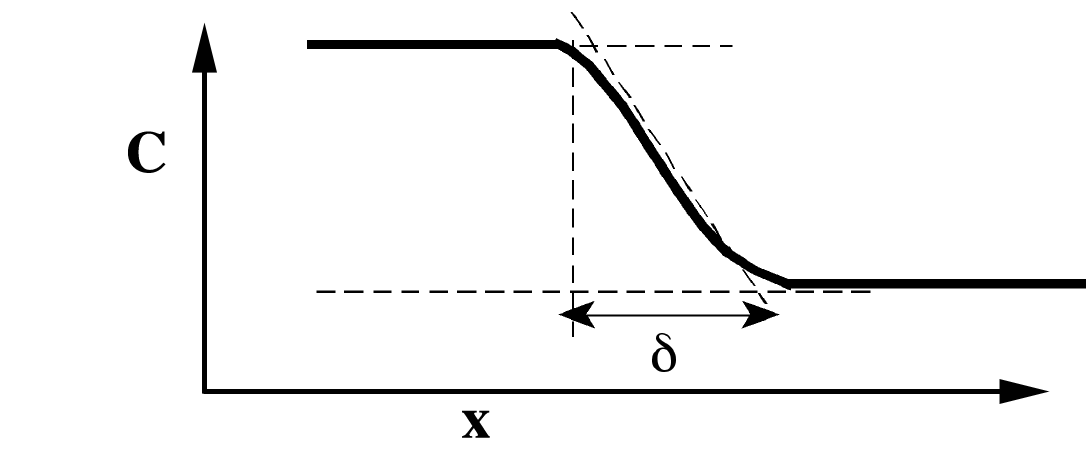
Fig. 1 Schematic of a diffuse interface.
A thermodynamically stable interface can only exist between two immiscible
fluids. Nonetheless, we use the term “miscible interface” to designate
the transition zone between two miscible fluids, which will necessarily relax
with
time. Stresses at a miscible interface were first explored by Korteweg [2].
He demonstrated that in the presence of a compositional gradient, stresses
would
result that would be analogous to stresses in immiscible fluids. Lowengrub
and Truskinovsky gave a thorough derivation of the Korteweg stress [3].
Zeldovich published an excellent work in which he showed that an interfacial
tension should exist between miscible fluids brought into contact [4]. Davis
proposed that when two miscible fluids are placed in contact they will immediately
begin to mix diffusively across the concentration front, and the composition
inhomogeneities can give rise to pressure anisotropies and to a tension between
the fluids [5]. Rousar and Nauman, following the work of Rowlinson and Widom
[6], proposed that an interfacial tension can be found without assuming that
the system is at equilibrium [7].
There have been several reports of phenomena in which the authors invoke an
interfacial tension with miscible systems [8-12]. Joseph and Renardy provided
a superb review
of the topic up to 1992 [13].
Pojman et al. provided definitive evidence for an effective interfacial tension
(EIT) in the isobutyric acid – water system [14]. They showed that after
a temperature jump above the Upper Critical Solution Temperature, a drop of
the IBA-rich phase persisted in a spinning drop tensiometer. The interfacial
tension
was on the order of 0.001 mN/m. Drops contracted when the rotation rate was
slowly decreased but drops broke up via the Rayleigh-Tomotika instability when
the rotation
rate was rapidly decreased. Lombardo et al. studied 1-butanol in water in which
the drop volume of 1-butanol was so small that the concentration was below
the solubility limit [15]. They also were able to measure an EIT.
The Korteweg term for miscible systems far from equilibrium can be compared
to Cahn and Hilliard’s treatment of immiscible fluids at equilibrium.
Using the Landau-Ginzburg free energy functional they were able to show that
the free
energy of a non-uniform immiscible system includes a term proportional to the
square of the concentration gradient [16]. This is precisely the same situation
Korteweg considered for miscible systems far from equilibrium.
For a system far from equilibrium the free energy functional can be written
as
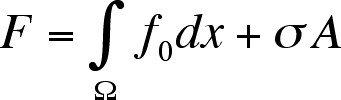
where f0 is the free energy of the uniform system and
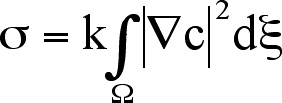
.
is the effective interfacial tension (EIT), and A the area of the interface.
This function can be reduced if we assume a linear concentration gradient.
The resulting equation for the EIT is
. 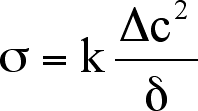
Here the EIT is written in terms of the Korteweg term, , henceforth referred
to as the square gradient parameter (with units of Newtons, N), the
change in composition , expressed as a mole fraction, and the width of the
transition
zone,
.
Chen and Meiburg have performed numerical simulations of miscible displacement
that included Korteweg stress [17-20]. Volpert et al. simulated convection
caused by curvature in drops in a miscible system [21]. Bessonov et
al. simulated Marangoni-like
convection for a miscible monomer-polymer system caused by concentration
and temperature gradients parallel to the transition zone between the
fluids [22-24].
The problem of testing these predictions on Earth is that buoyancy-driven
convection dominates the system. If a stream of honey is injected into
water, it will sink
because honey is denser than water. If a blob of water is injected
into honey, the water will float. Joseph and Renardy attempt to create
an
isopycnic system
with glycerol and sugar water [13]. However, diffusion always upset
the delicate balance between the densities of the fluids. Performing
experiments in weightlessness is an excellent approach to attempt to observe
behavior that can be attributed to Korteweg stress.
If no behavior
beyond diffusion is observed, it will allow the estimate of an upper
limit on the value of the square gradient parameter. We have previously
performed
experiments
with glycerol
and water on parabolic flights [21]. No convection was
observed but because of the short duration and poor quality of the
weightlessness, no conclusions could be drawn.
A major problem for predicting the potential importance to Korteweg
stresses in miscible fluids is estimating the value of the square
gradient parameter
for a pair of miscible fluids. By comparing simulations to the results
of this investigation,
we will be able to put an upper limit on the value of the square
gradient parameter for honey and water.
The Experiment -- MFMG
Back to the Main MFMG Page